Survey of Attitudes towards Trial sites in Europe
The SAT-EU StudyTM
Study Plan/Survey Protocol
The SAT-EU Survey is now complete.
Please click here for more information
Working Group
Marta Gehring1, Rod S. Taylor2, Brigitte Casteels3, Gian Franco Gensini MD4, Marie Mellody5 Giuseppe Ambrosio MD6
1 sbg Healthcare Consulting, Lausanne, Switzerland, 2Health Services Research, Peninsula Medical School, Universities of Exeter and Plymouth, UK, 3BC Consulting Geneva, Switzerland, 4Division of Cardiology, University of Florence, Florence, Italy, 6Virtuoso CRO Geneva, Switzerland, 7 Division of Cardiology, University of Perugia School of Medicine Perugia, Italy
Abstract
The European Commission and Industry task forces have explicitly stated Europe’s desire to maintain, and possibly develop its clinical trial competitiveness. A better understanding of Industry’s trial site selection criteria would be a useful tool in this respect.
While it is common wisdom in healthcare circles that speed of regulatory authorities’ decision-making is a key factor negatively influencing trial incidence in Europe, the relative weight of other important criteria is simply not known, nor is there any published information in this respect.
An independent task force, prompted and inspired by the existing Italian Network for Health Research (INHR) has been set up to run a survey of attitudes towards trial site selection in Europe. The SAT-EU StudyTM is a longitudinal, retrospective, observational and comparative study (survey) carried out in four stakeholder groups (drug companies, device manufacturers, CROs and academic clinical trial units) to assess the relative importance of different criteria in selecting trial sites for multinational phase II-III studies. The hypothesis is that criteria other than speed of regulatory approval may impact trial incidence. As such, the SAT-EU Study seeks to build on the work done by an FRP-7 supported consortium (ICREL) which effectively analyzed the impact on clinical research of the European Clinical Trial Directive (CTD)[1]
A well-kept secret can hardly be used to influence policies….
Background and Introduction
In 2010 Italy’s Ministry of Health created the Italian Network for Health Research (INHR) to foster Italy’s position in healthcare research, both overall and in innovative domains. The INHR’s mission is to facilitate early access to groundbreaking basic and translational research for Italian researchers, to increase collaboration between Italian and International Research Institutes, and to foster interaction with industry at the level of clinical research. Bringing more clinical trials to Italy may well fit within this framework.
On a larger scale, the Italian appeal for more health research is mirrored by Europe. A European Parliament 2009 policy paper regretted trial migration outside Europe, and found reasons for the phenomenon to include more rapid approval of trials and lower costs in non-EU countries[2]. Echoing this concern, the European Medicines Agency (EMA) reports that 65% of all data/patients in EU Market Authorisation submissions are already generated in third countries[3]. Numerable entities assign a good part of the blame to the Commission’s Clinical Trial Directive (CTD), which is said to place excessive bureaucratic burden and costs on European research (Directive 2001/20/EC). A Commissions task force estimated in March 2009 that executing just five of the 19 CTD’s key requirements would cost researchers Euros 715 Million per year[4].
Framework for the Study: Today’s Trial Incidence
It is in this context of Europe wanting to maintain its trial competitiveness that an independent task force was set up to run an observational and comparative study (survey) of attitudes towards trials sites in Europe. Ideas for its assembly were prompted by a brainstorming meeting organized by the Italian Network for Health Research (INHR) held in Cernobbio this past November 2010 and sponsored by Italy’s Ministry of Health (“Forum Ricerca”).[5]
Following presentations and discussions held at the Forum, authors of this document began by undertaking a feasibility/preliminary evaluation analysis of the issue. First, a survey of actual “trial incidence” was run across the following nine European countries: France, Germany, Italy, Spain, UK, Belgium, Netherlands, and Austria/Switzerland, including therefore Europe’s top five healthcare markets, and four smaller markets.
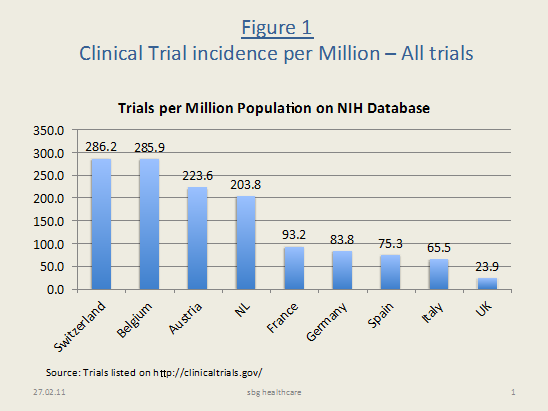
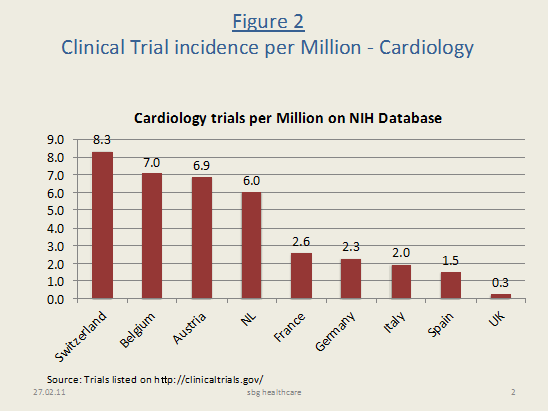
Sourcing studies listed on the US National Institute of Health (NIH) database,[6] our search revealed that Italy’s trial incidence per million ranks 8th (overall) and 7th (in Cardiology) out of these nine (see figures 1 and 2, pp. 7). Perhaps even more striking is that on a per capita basis Europe’s top five markets attract considerable less research than the smaller countries do. The trial incidence varies widely: from 286 trials per million in Switzerland and Belgium to 24 in the UK, taking trials for all therapeutic areas, and from 8.3 trials per million (Switzerland) to 0.3 (UK), for Cardiology specifically.
Clearly, not only can Europe do better, but so can Europe’s largest and most sought-after health-care markets: Italy, Germany, France, Spain and the UK.
Study Objectives
Multiple mechanisms can lead to a country’s underrepresentation in industry-sponsored clinical research. Some of them, which may prominently affect certain countries (e.g., lack of access to the internet, low patient load), are unlikely to play a major role in the case of large EU countries studied, such as Italy. Italy has gained a solid scientific reputation in Cardiology: Italian investigators regularly publish large numbers of papers in top Cardiology journals, and are among the most numerous attendees to International Cardiology meetings. Thus, limitations of scientific background and Principal Investigator English knowledge should be safely ruled out as contributing factors.
Accordingly, at this stage, the study group decided to focus on the specific issue of mechanisms of site selection. The task force’s immediate objective therefore, is to understand the key drivers impacting trial site selection in Italy and in Europe’s other top four markets and to rank potential improvements.
While it is common wisdom in healthcare circles that speed of Regulatory Authorities (MoH) and Ethical Committees (EC/IRB) decision-making are one of the key factors that may negatively influence trial incidence, the relative weight of numerous other criteria is simply not known. Interestingly, there is limited information available in this respect, both in the medical literature, and in health-care management intelligence databases.
The Study’s primary objectives are therefore (1) to understand Industry's trial site selection criteria for phase II -III multicentric, hospital based trials, looking at a total of 19 criteria in three categories (investigator, hospital and environment-driven criteria), and (2) to rank potential improvements across the three key domains (investigator, hospital and environment-driven) in Italy and in Europe. The secondary objective is to survey Industry's current perceptions of the Italian trial environment and hurdles to conducting clinical trials in Italy
Methods
In order to systematically evaluate key drivers impacting clinical trial site selection in Italy and in Europe, we have planned a four-step research-based process as follows:
Step I – List of criteria expected to impact trial site selection (done)
Step II – Development of appropriately designed Internet-based survey (done)
Step III - Undertake research based weighing of criteria in appropriate industry and academic sample
(underway Sept-October 2011)
Step IV - Analyze results and publish
Step I. Hypothesis on trial site selection criteria
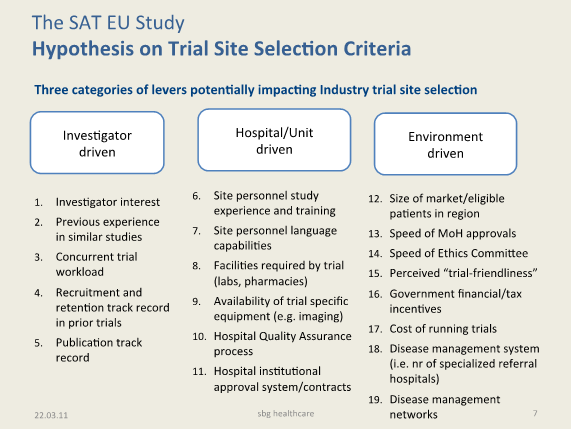
Criteria expected to impact trial site selection have been listed and organized in three broad categories: (a) Investigator-related, (b) Hospital/Institution related, and (c) Country/Environment related. The listed criteria will be validated with at least five experts (3 of which must be senior members of Clinical Research Organizations, CROs) to ensure that key criteria have not been missed, and/or that inconsequential items are taken off the list (see figure 5 below, “Hypothesis on Industry Trial Site Selection Criteria”).
The survey will weigh specified criteria by asking responders to divide 100 points across levers impacting clinical trial selection in each of a number of specified cases: by trial phase, and by type of lever (and if needed, by a combination thereof).
Step II. Internet based survey design
The second step involved building an easily accessible internet-based survey that will allow a wide range of respondents across Europe to provide their input (responsible: Marta Gehring). The survey has three specific objectives:
1. To understand Industry's trial site selection criteria for phase II -III multicentric, hospital based trials, looking at investigator, hospital and environment-driven criteria.
2. To survey Industry's current perceptions of the European and Italian trial environment and hurdles to conducting clinical trials
3. To rank potential improvements across the three key domains (investigator, hospital and environment-driven)
A draft of the survey has been built and currently stands at 26 questions. As set up, the survey (a) obtains responder background information in a blinded fashion (b) weighs the relevance of each of the hypothesized trial site selection criteria for Europe as a whole (c) surveys current perception of the Italian trial environment, and (d) ranks potential improvements, focusing on Italian Cardiology
The survey is hosted by “Survey-Monkey”, an online software and questionnaire tool provided to respondents at no cost to them (www.surveymonkey.net). A health-care market research expert has reviewed the survey to help minimize possible bias in question content and organization (the Planning Shop International in London; http://www.planningshopintl.com/)
The survey leverages various question types, most important of which are: single or multiple choice and ranking/weighing of criteria.
Step III. Research based weighing of criteria in appropriate industry sample
The study group will aim to recruit at least 150 non-remunerated volunteer respondents from at least 30 different institutions, including pharmaceutical companies, device manufacturers, CROs and academic CTUs. Since we expect a 10% response rate, we will plan our sample of survey-response requests accordingly.
In order to ensure adequate response rates across Europe’s Health-Care Industry, we have gratefully obtained the collaboration of the following, Industry and Clinical trial Associations, and online communities:
European Biotech Industry Association
http://www.europabio.org/eu_index.htm
European Pharmaceutical Industry Association
http://www.efpia.org/Content/Default.asp
Applied Clinical Trials
http://appliedclinicaltrialsonline.findpharma.com
Pharma IQ
Drug Information Association (DIA)
http://www.diahome.org/DIAHome/Home.aspx
A pilot survey was successfully conducted in June 2011 in order to validate the survey questions and refine the search. The SAT-EU survey then ran between Monday September 26 and Monday October 31st, collecting 372 responders. The Survey was then re-opened again to allow for EFPIA’s participation which is running from December 16th to early January 2011. All interested participating organizations will have first access to results.
Step IV. Analysis and Publication
In this last step, the study group will review results in two ways. First, it will compile summary outcomes and analyze survey data using software provided by the SurveyMonkey tool. Secondly, it will perform additional ad-hoc analysis as may be relevant, based on survey results (to be discussed by the project team).
Following analysis, the study group will meet to review results, derive implications, and plan appropriate peer-reviewed publication in target journal.
With this information in hand, the study group, its advisors and network partners will be able to decide how the information could best be used to influence any of the identified criteria from “easiest” to “hardest” to impact: investigator, hospital, and policy driven/legislative criteria.
Project Relevance
To the best of our knowledge, there is no survey on Industry’s trial site selection criteria available. Neither is there any published information on the topic, whether from the medical or the management literature. Accordingly, we believe that results of such a survey may interest not only Industry Associations, CROs, and CTUs, but might also be of potential use to the European Commission in Brussels and the European Medicines Agency (EMA) in London.
List of Figures
|
Figure |
|
Figure 1: Clinical Trial incidence per Million – All trials on NIH database (Oct 2010) |
|
Figure 2: Clinical Trial incidence per Million – Cardiology trials on NIH Database (Oct 2010) |
|
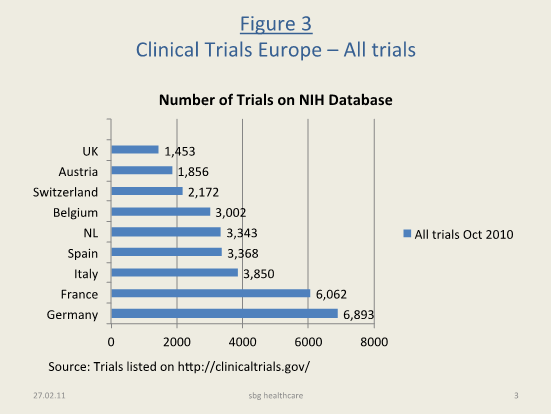
Figure 3: Clinical Trials in Europe – All trials on NIH database (Oct 2010) |
|
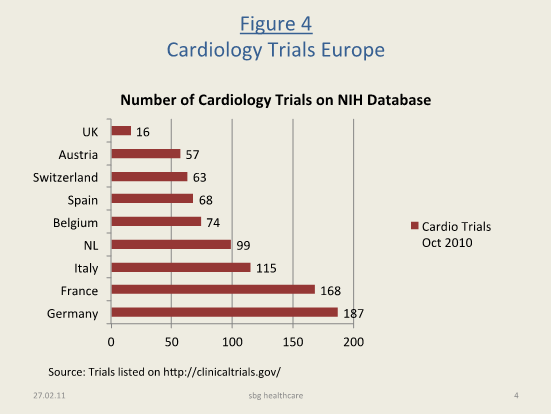
Figure 4: Cardiology Trials in Europe (NIH database, Oct 2010) |
|
Figure 5: Hypothesis on Industry Trial Site Selection Criteria (for testing) |
Note that trial incidence figures are currently under validation with several clinical trial databases, including “Controlled Trials” (www.controlled-trials.com) and EudraCT’s public site now in population (www.clinicaltrialsregister.eu/ctr-search/). However, the study group has thus far found the US NIH’s ClinicitalTrials.gov to be the most representative database. Hence trial incidence figures shown in this working document are based on clinical trials listed on http://clinicaltrials.gov/